Does Your CV Pass the 6-Second Rule? How to Get Seen in the Pharmaceutical & Life Science Job Market
In my years of recruiting within the pharmaceutical, biotech, and life sciences sectors, I’ve learned one cold truth:
A hiring manager can decide in just 6 seconds whether to keep reading your CV or resume - or move on.
Sometimes, that decision is made even earlier, because your CV never makes it past the Applicant Tracking System (ATS) in the first place.
Think of your CV like a luxury store window: it should instantly showcase your most valuable skills, qualifications, and achievements for that role. A cluttered, poorly formatted, or generic CV is like a dusty shop window - people walk past without looking twice.
Step 1: Understand the ATS Barrier
In most large pharmaceutical companies, including Johnson & Johnson, Merck & Co., Novartis, and Sanofi, your CV will first be scanned by an ATS before it’s ever read by a human.
- 75–80% of CVs are rejected by ATS before reaching a recruiter’s desk (Source: Jobscan, 2024).
- ATS software filters CVs based on keywords, job title matches, and formatting.
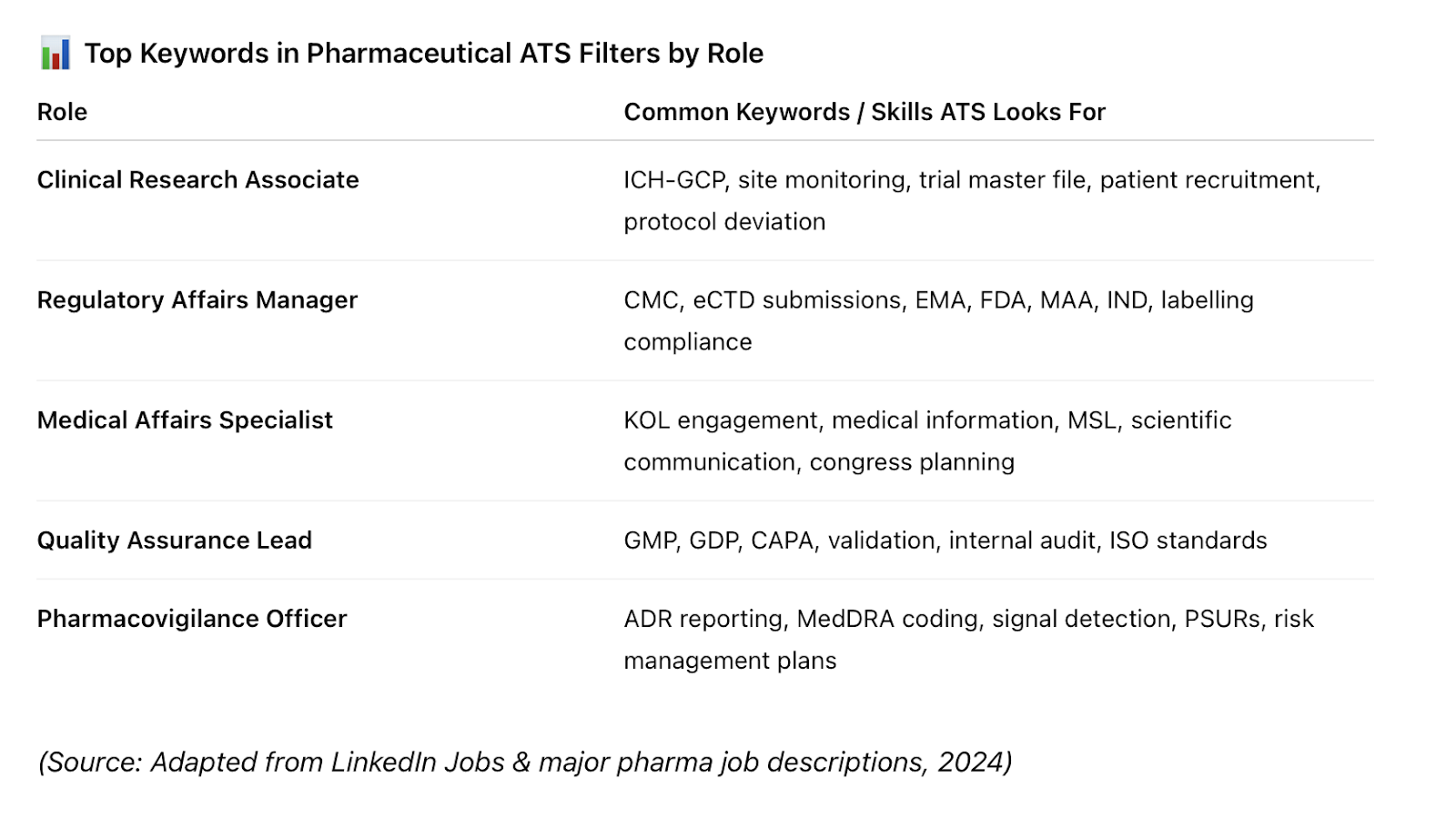
- In the pharma industry, specific role-related terms (e.g., ICH-GCP, CMC development, oncology trials, pharmacovigilance) are critical.
💡 Tip: Always adapt your CV to match the exact language in the job description, especially for niche pharma and medical terms and skills.
Step 2: Pass the 6-Second Human Scan
Once your CV gets through the ATS, it lands in front of a recruiter or HR manager.
Research from TheLadders shows recruiters spend just 6 seconds deciding whether to keep reading.
Here’s what they’re looking for in those precious seconds:
- Relevant, recent job titles that align with the role.
- Key achievements that show impact, not just responsibilities.
- Clear qualifications & certifications that prove sector expertise.
💡 Tip: Place your strongest, most relevant content in the top half of page one. This is prime real estate.
Step 3: Tailor for the Pharmaceutical & Life Sciences Audience
Whether you’re applying for a clinical research role at GSK, a regulatory affairs position at Bristol Myers Squibb, or a manufacturing leadership role at Eli Lilly, your CV must show you understand the industry’s priorities.
The sector values:
- Regulatory knowledge (FDA, EMA, ICH guidelines)
- Therapeutic expertise (oncology, rare diseases, vaccines, infectious diseases)
- Technical skills (bioinformatics, lab automation, clinical data analysis)
- Compliance & quality (GMP, GDP, pharmacovigilance)
- Cross-functional teamwork (R&D, regulatory, manufacturing, marketing)
💡 Tip: If you’ve worked in global or cross-border roles, make sure this is visible in your summary or key achievements.
Step 4: Avoid the Common CV Killers in Recruitment
- Generic CVs - One-size-fits-all rarely works.
- Overuse of jargon without clarity - Use technical terms but make them understandable to non-specialists.
- Too much “responsible for” - Focus on results, not just duties.
- Formatting ATS can’t read - Overly complex layouts with tables, text boxes, or graphics often fail ATS scans.
Step 5: Leverage Tools that Optimise for ATS and Humans
Even the best content can fail if ATS can’t read it. That’s why we launched pharmaCV.com, powered by Rezi’s ATS-optimised CV technology.
- ATS score check – See how your CV matches the job description.
- Keyword optimisation - Ensure you’re speaking the language recruiters search for.
- Industry-tailored templates - Designed for pharma, biotech, and life sciences roles.
But remember, an ATS-friendly CV is only the starting point. Your story, achievements, and clarity still win the day when a real person reads it.

Key Takeaway:
If your CV doesn’t pass the ATS filter and the 6-second recruiter test, it won’t reach the decision-maker, whether that’s at Pfizer, Roche, GSK, AstraZeneca, or any other leading employer. Make it relevant, keyword-rich, visually clear, and achievement-driven.
And if you want a head start, pharmaCV.com will help you create a CV that works in both worlds - technology and human judgment.
-1.png)
.webp)



